Article in the Journal of Physical Chemistry C
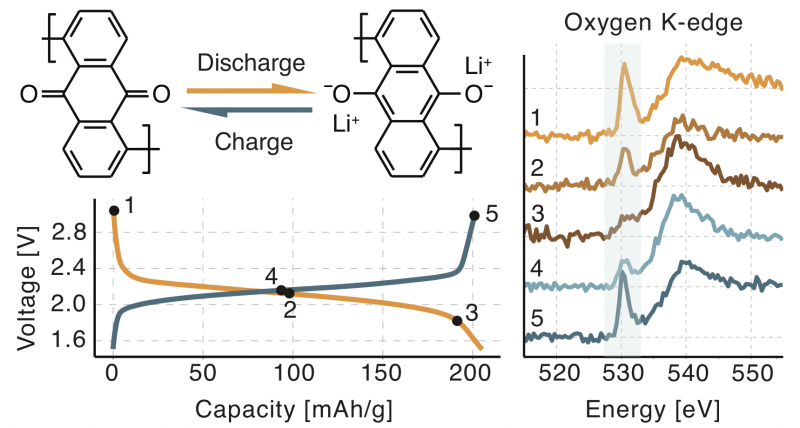
The electrochemical activity of metal-organic batteries is a result of a reversible reduction of a double carbonyl bond. To characterize the electrochemical process, X-ray Raman spectroscopy was used to measure the oxygen K-edge absorption spectra of organic polymer cathodes from different multivalent metal−organic batteries.The presence of a carbonyl bond is easily identified in the absorption spectra by the characteristic resonance at 530 eV, which was confirmed to originate from the 1s to π* electronic transition. Relative amounts of carbonyl bond in samples were determined and used to quantify the rate of the electrochemical reaction in cathode samples.
The samples were prepared by the researchers at the National Institute of Chemistry, XRS measurements were carried out at the P01 beamline of PETRA III synchrotron facility in Hamburg.